Viral Vector Process Development
The Viral Vector Process Development team focuses on developing GMP-compliant production processes and innovative technologies to constantly improve process performance.
Activities & Services
- Expertise in AAV (various serotypes) and LV
- Two efficient production platforms:
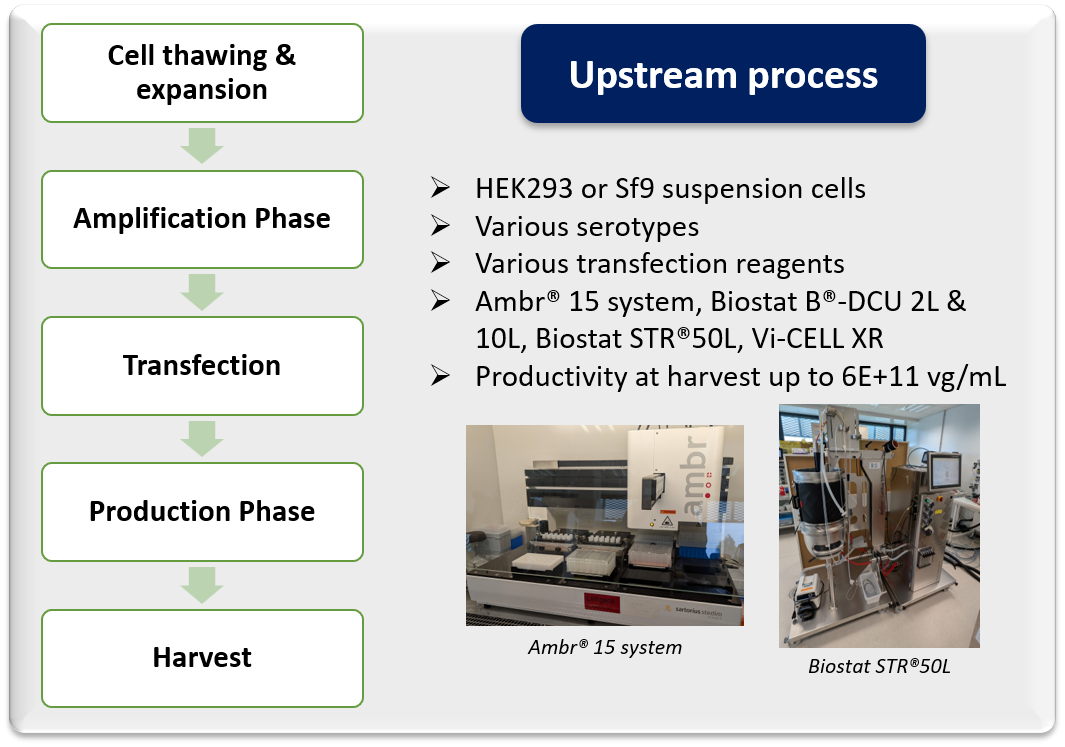
- Transient transfection of HEK293 cells in suspension
- Infection of Sf9 cells in suspension with two Baculoviruses (BEVs system)
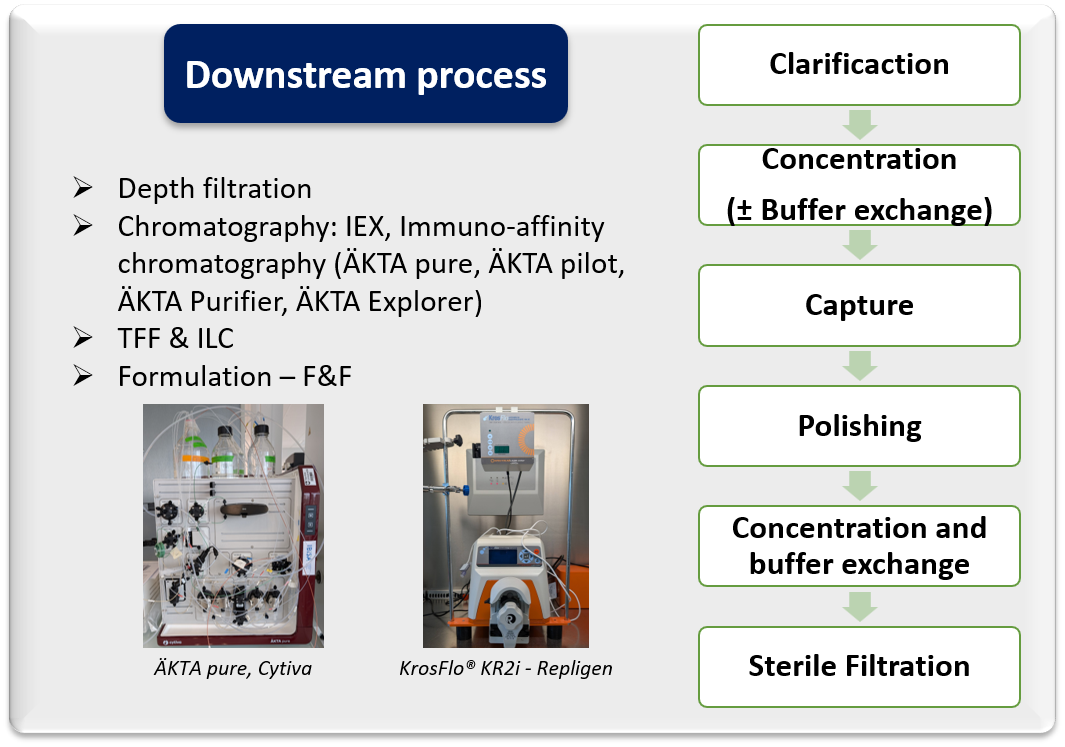
- Custom upstream and downstream process development and optimization
- Feasibility study at small scale
- Process scale-up to pilot scale (50L)
- Assessment of innovative upstream and downstream technologies
- Deep process characterization
- Manufacturing of non-GMP materials
- Technology transfer to CMO

Process development experts at your service to set up and improve your manufacturing process !
Mis à jour le 30 août 2024.