Preclinical Analytics Core (PAC)
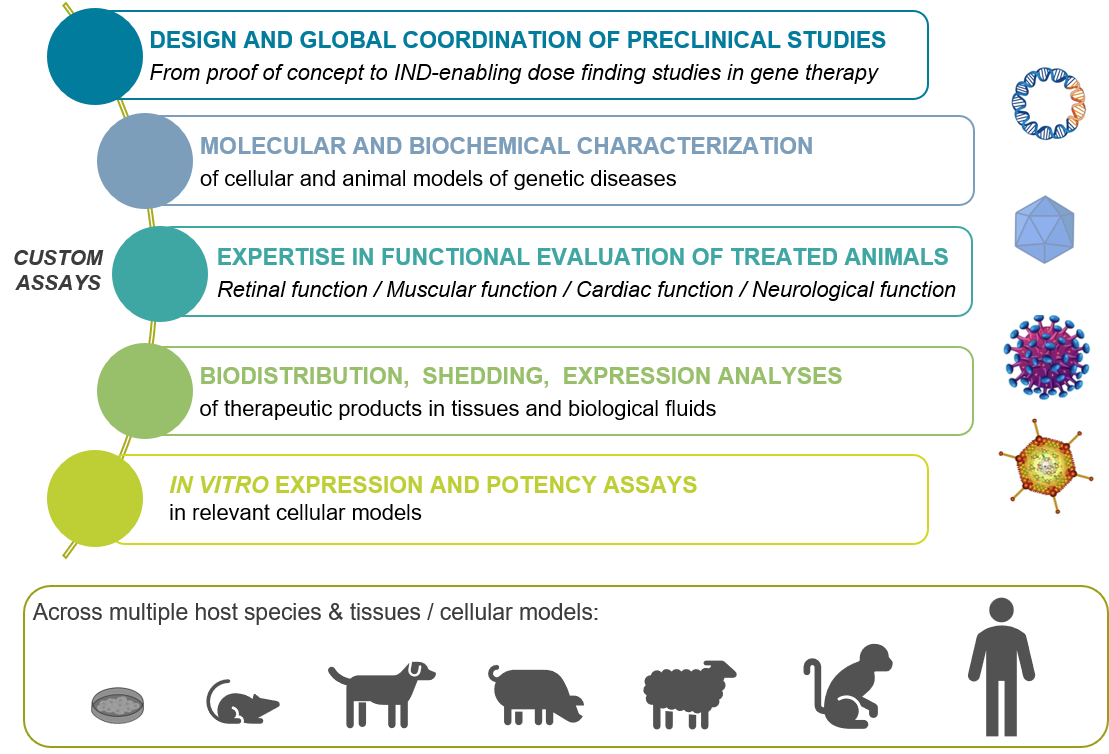
The Preclinical Analytics Core provides skilled expertise in the management (including design and global coordination) of gene therapy preclinical trials in animal models. The team has participated to a large number of preclinical studies in rodent, canine, pig, sheep and nonhuman primate models, from proof of concept studies to IND-enabling dose findings as well as in toxicological gene therapy studies.
The core also offers a large panel of assays to perform :
- Molecular and biochemical characterization of cellular and animal models of genetic diseases, including specific genotyping of animal models with genetic diseases.
- Therapeutic product administration, and functional evaluation of treated animals (retinal function, muscular function, cardiac function, neurological function)
- Gene therapy product biodistribution and shedding analysis in tissues and biological fluids (absolute quantification of the transgenic genome) as well as gene therapy product expression analysis in tissues (relative quantification of transgenic messenger and specific detection of the transgenic protein). Such analysis are required to understand the pharmacology and the pharmacokinetic of a therapeutic product, i.e. to determine the presence, the persistence and the expression level of this product within the targeted tissue, but also its dissemination into non-targeted tissues and biological fluids.
- Specific in vitro expression / potency assays for quality control release of a gene therapy product, after transduction of specific cell and relevant molecular / biochemical analysis.
All preclinical analytics services are running under an ISO 9001, version 2015, approved quality system. All studies are performed to guarantee data and sample traceability. Validated procedures and approved documents are used for all types of analysis.
Equipment of the core PAC include :
- Automated liquid handler station
- Tissue homogenizer
- Nanophotometer
- Real-time PCR system
- Digital PCR system
- Microfluidic-based nucleic acid analyzer
- Trans-turbo transfer system
- High resolution Western-blot Imaging System
- Automated Simple Western System
- Microplate washer
- ELISA microplate reader
- ECLIA system
- Surgical microscope for retinal injections
- Eye Fundus /OCT machine
- ERG system (including multifocal ERG)

dPCR

MSD reader

Jess
The core is supervised by Dr Caroline Le Guiner, PhD and has dedicated human resources (1 full time technician and 2 part time engineers).


Contact
Scientific Advisor
caroline.le-guiner@univ-nantes.fr
Audrey Bourdon, PhD
Operational Manager
audrey.bourdon@univ-nantes.fr