GTI Core
Monitoring host immune responses in gene therapy studies
Closely linked to the U1089 research team Immunology for gene transfer , the Gene Therapy Immunology core (GTI) provides skilled expertise in the monitoring of host immunity in preclinical and clinical gene therapy protocols.
A wide panel of analysis
We propose various analysis for the monitoring of immune response against the vector and the transgene product.
Humoral immunity
- Viral neutralizing factors (AAV, Adenovirus)
- Antibody detection (ELISA, Western Blot)
- Cytokine quantification


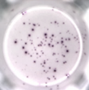
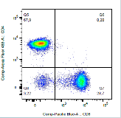
Cellular Immunity
- T cell response assessment :
- ELISpot
- Cell proliferation assay
- Multimer
- Multiparametric cell phenotyping (flow cytometry)
Various analysis settings
Our assays are avalaible for multiple vectors:
- AAV1, AAV2, AAV3, AAV4, AAV5, AAV6, AAV8, AAV9 et AAV10
- Adenovirus
- Mouse
- Rat
- Dog
- Monkey
- Human
Sample analyzed
- PBMC (Peripheral Blood Mononuclear Cells)
- Splenocytes
- Lymph node cells
- Plasma
- Serum
- Cerebro Spinal Liquid
- Plasma /serum isolation from whole blood
- Cell isolation from :
- Whole blood (PBMC)
- Spleen
- Lymph nodes
- Bone marrow
From research studies to clinical trials
The GTI core is involved at the different steps of product development from research and pilot studies to regulatory studies: toxicology and clinical trial. The activity is submitted to the quality management system approved by LRQA certification to ISO 9001:2015 and applies Good Clinical laboratory Practices (GCLP) for clinical trials monitoring.


Contact
- Scientific head :
- Operational manager :