TaRGeT - UMR1089
TaRGeT laboratory is based in Nantes Université, at the 4th and 5th floors of “Nantes Biotech/Institut de Recherche en Santé 2 (IRS2)" building.
UMR 1089 (before known as U649) is an internationally recognized expert in the field of gene therapy using viral vectors, in particular in Adeno-Associated Virus-derived vectors (AAV).
The lab has put together a large number of scientific and technological skilled expertise to advance AAV gene therapy products to the clinic among which:
- vector design and engineering,
- vector preclinical evaluation in neuromuscular and retinal diseases,
- vector immunogenicity and
- vector large scale bioproduction.

IRS2 Nantes Biotech building
It is composed in 6 groups and 3 core facilities working together, closely helped by a quality management support :
The Translational vector core ViVeM (ex CPV) created in 1997
has been labelled by the French government in 2020 as one among the 6 national industrial integrators
of the “Grand Défi National des Biomédicaments”.
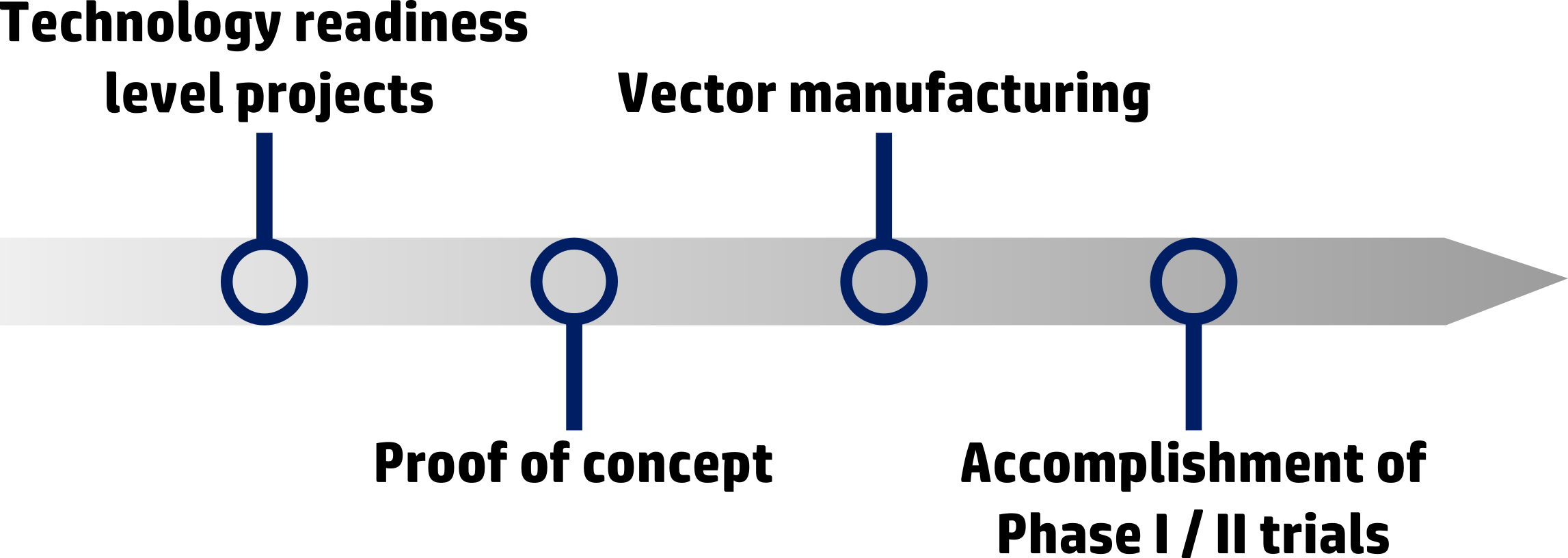
The laboratory offers a unique setting in France able to cover the entire translational gene therapy development path :
Since 2004, the whole laboratory activities are performed under a quality management system that is approved by Lloyd's Register Quality Assurance LRQA to meet requirements of international Management System Standards ISO 9001:2015.

Mis à jour le 06 September 2024.